Tag Archives: Blood Warming
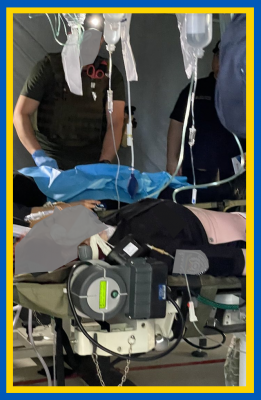
Warrior <> Ukraine
We are fortunate to work with amazing people and great organizations that share the same [...]
Jun
The Perfect Backdrop To Unveil Innovation
Norway’s fjords and the THOR conference are the perfect backdrop to unveil the future of blood and [...]
Jun
QinFlow and Life-Assist Complete Onboarding of Distribution Partnership for Warrior Blood and IV Fluid Warmers
PLANO, Texas – June 16, 2023 – QinFlow Inc. and Life-Assist have completed integration of a distribution partnership for the Warrior line [...]
Jun
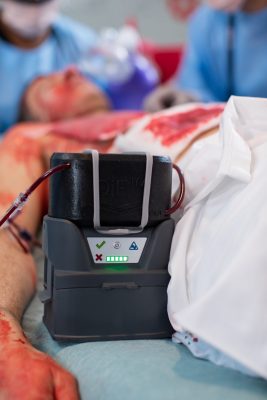
Your Agency Can Reduce 30-Day Mortality by 37% Simply by Implementing Prehospital Blood Delivery, Study Says
Nearly a third of those who die from traumatic injury do so from bleeding.2 Delivery [...]
Apr
The Prehospital Blood Train Has Left the Station!
The prehospital blood program train in the U.S. has left the station! We estimate >300 [...]
Apr
Thank You TraumaCon!
Thanks to all who came by our booth at #TraumaCon2023 to learn about the Warrior line of [...]
Mar
Florida Whole Blood Coalition Is Back!
If your institution is interested in carrying whole blood, this is a program you don’t [...]
Mar
410 Medical & QinFlow Share Booth at MAEMSP Conference
Great to see everyone at #NAEMSP23 in discussing delivery of warm fluids and blood for the continuum [...]
Jan
Aluminum: The Hidden Danger in Blood Warmers
Recently, there have been growing concerns from regulatory agencies, such as the FDA, as to [...]
Sep
Does Your Warmer Handle Intermittent (Bolus) Flows?
In a previous article I discussed the top ten things you need to look for [...]
Sep
- 1
- 2