Category Archives: General
Introducing QinFlow’s Warrior lite AC: The Ultimate Blood & Fluid Warming Solution for Hospital Settings
Global Press Release Distribution: LONDON – Sept. 9, 2024 – QinFlow announces the launch of [...]
Sep
Warrior lite AC Is Now Cleared for Distribution*
🚀 Exciting News! We’re thrilled to introduce the Warrior lite AC, our latest innovation in [...]
Sep
Whole Blood In Rural EMS: The Wilkes County Story
Peter Antevy and the NAEMSP Florida Chapter host a webinar with Jeff W. Hinshaw, MS, [...]
Aug
Warming Blood Makes A Difference for Trauma Patients!
Hypothermia is common in trauma patients, with approximately 40% to 50% of moderate to severely [...]
Aug
EMS World Expo Is Coming Soon…
If you are planning to be at EMS World Expo, we invite you to Booth [...]
Aug
ENA 24 Is Fast Approaching!
ENA 24 is fast approaching! Come visit booth 432 to discover what Dignity Health, Atrium [...]
Aug
SPARC Academy Is Sparking Alaska…
Text and Image Credit: Randi Schaefer SPARC Academy Alaska was a great success! We were [...]
Aug
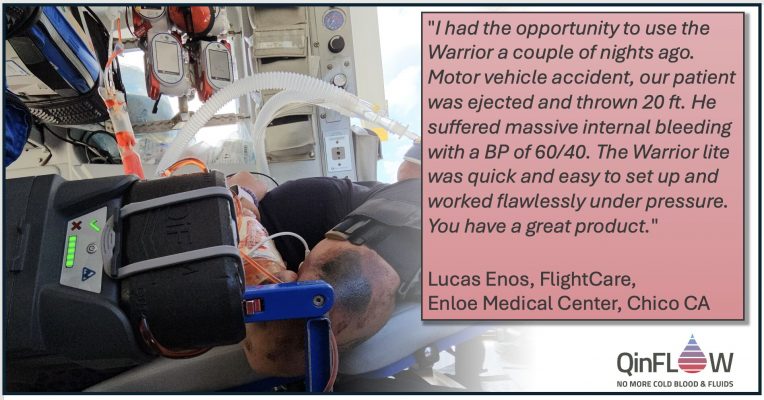
Exciting Testimonial from Enloe Medical Center, California
Testimonials such as this one from Enloe Medical Center in California reinforce our passion to [...]
Aug
“Removing the Barriers To Prehospital Blood: A Roadmap To Success”
Credit: Schaefer, Randall M. DNP; Bank, Eric A. AS; Krohmer, Jon R. MD; Haskell, Andrew [...]
Aug
Frustrated with Blood Warmers Consumable Backorders?
Equip your team with the Warrior line of blood and fluid warmers, designed to keep [...]
Aug